Moles and Chemical Formulas Report Sheet: Moles and Chemical Formulas Date _________ _ _ Section ____ _ _____ _ Instructor _________ _ Name Team A. Finding the Simplest Formula A.I Mass of empty crucible+ cover A.2 Initial appearance of magnesium Mass of crncible +cover+ magnesium _ ____________ g _ ___ _ __ _ _ _ _ __ g A.3 Mass of crucible+ co
Answered: REPORT SHEET LAB Moles and Chemical… | bartleby
This worksheet is designed to refresh your memory of simple mole calculations and give you opportunities to use these tools to solve more complex problems. Moles, mass, and relative formula mass. The relationship between moles, mass and Mr can be represented by this equation: Part 1: Working out the moles from the mass of a known substance.
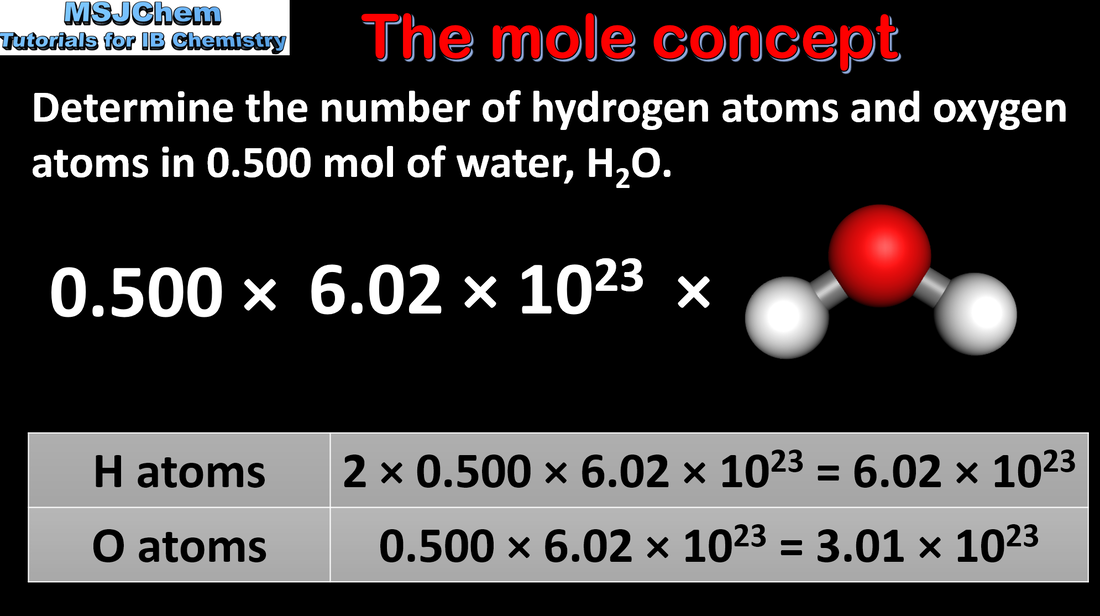
Source Image: msjchem.com
Download Image
Compounds that are ionic, like NaCl, are represented by ionic formulas. One mole of NaCl, for example, refers to 6.022 × 10 23 formula units of NaCl. And, one formula unit of NaCl consists of one sodium ion and one chloride ion. Figure 6.1.2 summarizes the basic units of elements, covalent and ionic compounds
![physical science 20] moles : r/HomeworkHelp](https://i.redd.it/vke7wpe4vcqb1.jpg)
Source Image: reddit.com
Download Image
IB Chemistry Serial Dilution, Molarity and Concentration | PPT
Study with Quizlet and memorize flashcards containing terms like Using the rules for writing the formulas of ionic compounds, write the ions of the correct formula for magnesium oxide., Write a balanced equation for the reaction of the magnesium and the oxygen (O2), including their physical states, Calculate the simplest formula for the following compound: a. 0.200 mole of Al and .600 mole of

Source Image: reddit.com
Download Image
Moles And Chemical Formulas Report Sheet Answers
Study with Quizlet and memorize flashcards containing terms like Using the rules for writing the formulas of ionic compounds, write the ions of the correct formula for magnesium oxide., Write a balanced equation for the reaction of the magnesium and the oxygen (O2), including their physical states, Calculate the simplest formula for the following compound: a. 0.200 mole of Al and .600 mole of
In the previous section, several relationships were written, including:. 1 mol Al = 26.98 g Al = 6.022 × 10 23 atoms Al ; 1 mol C 12 H 22 O 11 = 342.3 g C 12 H 22 O 11 = 6.022 × 10 23 molecules C 12 H 22 O 11; 1 mol NaCl = 58.44 g NaCl = 6.022 × 10 23 formula units NaCl; These relationships may be used to convert from grams to moles or vice versa; or from moles to atoms, molecules, or
Writing formulas for ionic compounds. Why do I start off with 2+ and 2-, but end with 1+ and 1- in final answer? : r/chemhelp
A lab performed in Chemistry determining chemical formulas lab report sheet date: name: kb bohm lab partner(s): jared purpose: prepare for the lab writing … mass of evaporated water was 0 g mass percent of evaporated water was 35 % during the experiment. moles of anhydrous copper sulfate was 0. moles of evaporated water was 0. empirical
SOLVED: REPORT SHEET LAB: Moles and Chemical Formulas Finding the Simplest Formula Mass of empty crucible + cover: 236.23 g Initial appearance of the magnesium: 2.012 g Mass of crucible + cover +
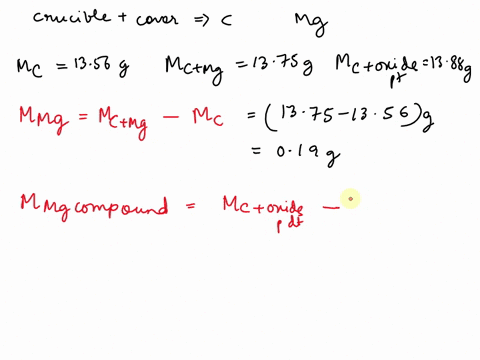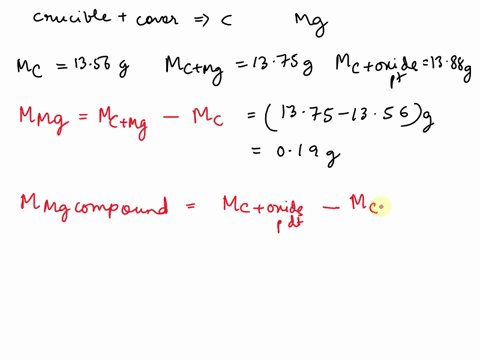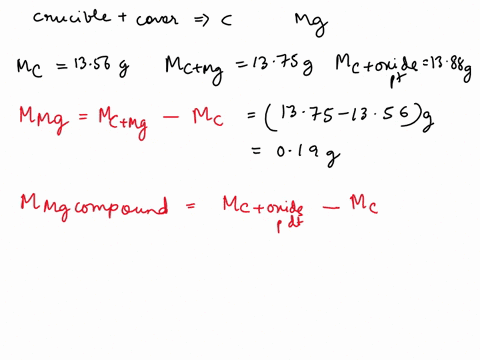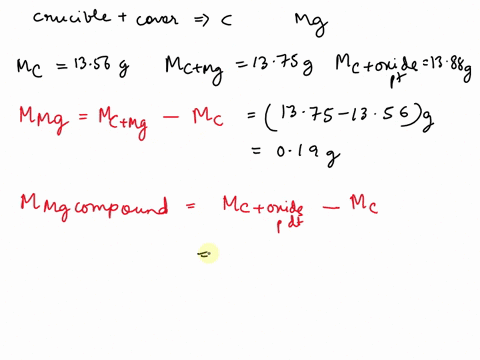
Source Image: numerade.com
Download Image
Chemsheets GCSE 1092 Moles ANS | PDF | Mole (Unit) | Chemistry
A lab performed in Chemistry determining chemical formulas lab report sheet date: name: kb bohm lab partner(s): jared purpose: prepare for the lab writing … mass of evaporated water was 0 g mass percent of evaporated water was 35 % during the experiment. moles of anhydrous copper sulfate was 0. moles of evaporated water was 0. empirical

Source Image: es.scribd.com
Download Image
Answered: REPORT SHEET LAB Moles and Chemical… | bartleby
Moles and Chemical Formulas Report Sheet: Moles and Chemical Formulas Date _________ _ _ Section ____ _ _____ _ Instructor _________ _ Name Team A. Finding the Simplest Formula A.I Mass of empty crucible+ cover A.2 Initial appearance of magnesium Mass of crncible +cover+ magnesium _ ____________ g _ ___ _ __ _ _ _ _ __ g A.3 Mass of crucible+ co

Source Image: bartleby.com
Download Image
IB Chemistry Serial Dilution, Molarity and Concentration | PPT
Compounds that are ionic, like NaCl, are represented by ionic formulas. One mole of NaCl, for example, refers to 6.022 × 10 23 formula units of NaCl. And, one formula unit of NaCl consists of one sodium ion and one chloride ion. Figure 6.1.2 summarizes the basic units of elements, covalent and ionic compounds

Source Image: slideshare.net
Download Image
Master the Periodic Table with ChatGPT: Effortless revision for any topic
masses of the atoms in the chemical formulae. Relative formula mass: in many ways this is more accurate than Relative Molecular Mass. Many salts, even in the solid state, exist as ions rather than molecules. Although the formula of sodium chloride is normally given as NaCl, it is not a simple molecule but a giant lattice and it is more

Source Image: geniebook.com
Download Image
Group 2, The Alkaline Earth Metals (A-Level Chemistry) – Study Mind
Study with Quizlet and memorize flashcards containing terms like Using the rules for writing the formulas of ionic compounds, write the ions of the correct formula for magnesium oxide., Write a balanced equation for the reaction of the magnesium and the oxygen (O2), including their physical states, Calculate the simplest formula for the following compound: a. 0.200 mole of Al and .600 mole of
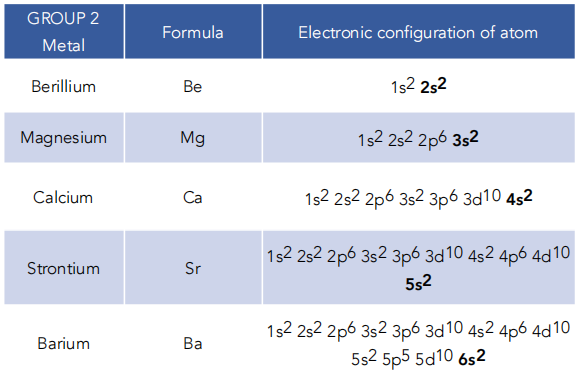
Source Image: studymind.co.uk
Download Image
Lab 11-Moles and Chemical Formulas-Lab Report.doc – A. Finding the Simplest Formula REPORT SHEET Moles and Chemical Formulas LAB 1. Mass of empt 11 2. | Course Hero
In the previous section, several relationships were written, including:. 1 mol Al = 26.98 g Al = 6.022 × 10 23 atoms Al ; 1 mol C 12 H 22 O 11 = 342.3 g C 12 H 22 O 11 = 6.022 × 10 23 molecules C 12 H 22 O 11; 1 mol NaCl = 58.44 g NaCl = 6.022 × 10 23 formula units NaCl; These relationships may be used to convert from grams to moles or vice versa; or from moles to atoms, molecules, or

Source Image: coursehero.com
Download Image
Chemsheets GCSE 1092 Moles ANS | PDF | Mole (Unit) | Chemistry
Lab 11-Moles and Chemical Formulas-Lab Report.doc – A. Finding the Simplest Formula REPORT SHEET Moles and Chemical Formulas LAB 1. Mass of empt 11 2. | Course Hero
This worksheet is designed to refresh your memory of simple mole calculations and give you opportunities to use these tools to solve more complex problems. Moles, mass, and relative formula mass. The relationship between moles, mass and Mr can be represented by this equation: Part 1: Working out the moles from the mass of a known substance.
IB Chemistry Serial Dilution, Molarity and Concentration | PPT Group 2, The Alkaline Earth Metals (A-Level Chemistry) – Study Mind
masses of the atoms in the chemical formulae. Relative formula mass: in many ways this is more accurate than Relative Molecular Mass. Many salts, even in the solid state, exist as ions rather than molecules. Although the formula of sodium chloride is normally given as NaCl, it is not a simple molecule but a giant lattice and it is more