Notice that 3‑methylhexane is one word. … Halogen substituents are easily accommodated, using the names: fluoro (F-), chloro (Cl-), bromo (Br-) and iodo (I-). Example 3.4.2 : Halogen Substitution. For example, (CH 3) 2 CHCH 2 CH 2 Br would be named 1-bromo-3-methylbutane. If the halogen is bonded to a simple alkyl group an alternative
5-Bromo-2-chloro-4-fluoro-3-iodopyridine as a Halogen-rich Intermediate for the Synthesis of Pentasubstituted Pyridines | The Journal of Organic Chemistry
About Transcript Common and IUPAC nomenclature of alkyl halides. Classifying primary, secondary, and tertiary alkyl halides. Created by Jay. Questions Tips & Thanks Want to join the conversation? Sort by: Top Voted Zoe 11 years ago At about 6:30 , when applying R and S to 2-iodo-3-methylpentane, why isn’t the number 3 carbon chiral as well?

Source Image: sigmaaldrich.com
Download Image
A pair of theoretical enantiomers is named below. For each stereoisomers, draw the structure. Be sure to specify the stereochemistry via wedge-and-dash bonds. (S)-5,5-dibromo-3-fluoro-2-methyl-3-hexanol and (R)-5,5-dibromo-3-fluoro-2-methyl-3-hexanol; Draw the gauche conformer of the following compound looking down the indicated bond.

Source Image: hsppharma.com
Download Image
3-Bromo-5-chloro-2-methylaniline | Sigma-Aldrich May 25, 2023c Add wedge and dash bonds to give 3R 5 bromo 2 chloro 3. Last updated: 5/25/2023. c Add wedge and dash bonds to give 3R 5 bromo 2 chloro 3 fluoro 3 methylhexane Indicate the configuration at the 3 position only The configurations at the 2 and 5 positions are unspecified those groups are used to determine priorities for the configuration at the
Source Image: pubchem.ncbi.nlm.nih.gov
Download Image
3r 5 Bromo 2 Chloro 3 Fluoro 3 Methylhexane
May 25, 2023c Add wedge and dash bonds to give 3R 5 bromo 2 chloro 3. Last updated: 5/25/2023. c Add wedge and dash bonds to give 3R 5 bromo 2 chloro 3 fluoro 3 methylhexane Indicate the configuration at the 3 position only The configurations at the 2 and 5 positions are unspecified those groups are used to determine priorities for the configuration at the c. Add wedgeand-dash bonds to give (3R)-5-bromo-2-chloro-3-fluoro-3-methylhexane. Indicate the configuration at the 3-position only (The configurations at the 2– and 5-positions are unspecified; those groups are used to determine priorities for the configuration at the 3-position.) Not the question you’re looking for?
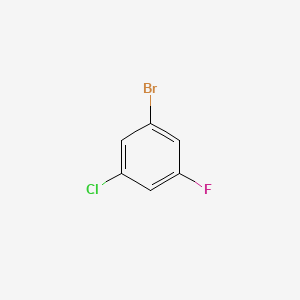
1-Bromo-3-chloro-5-fluorobenzene | C6H3BrClF | CID 2736223 – PubChem
Science Chemistry c. Add wedge-and-dash bonds to give (3R)-5-bromo-2-chloro-3-fluoro-3-methylhexane. Indicate the configuration at the 3-position only. (The configurations at the 2– and 5-positions are unspecified; those groups are used to determine priorities for the configuration at the 3-position.) Select Draw Rings More Erase H Br Cl 3-Bromo-2-fluoro-2-methylpentane – SpectraBase

Source Image: spectrabase.com
Download Image
Search Science Chemistry c. Add wedge-and-dash bonds to give (3R)-5-bromo-2-chloro-3-fluoro-3-methylhexane. Indicate the configuration at the 3-position only. (The configurations at the 2– and 5-positions are unspecified; those groups are used to determine priorities for the configuration at the 3-position.) Select Draw Rings More Erase H Br Cl

Source Image: simsonpharma.com
Download Image
5-Bromo-2-chloro-4-fluoro-3-iodopyridine as a Halogen-rich Intermediate for the Synthesis of Pentasubstituted Pyridines | The Journal of Organic Chemistry Notice that 3‑methylhexane is one word. … Halogen substituents are easily accommodated, using the names: fluoro (F-), chloro (Cl-), bromo (Br-) and iodo (I-). Example 3.4.2 : Halogen Substitution. For example, (CH 3) 2 CHCH 2 CH 2 Br would be named 1-bromo-3-methylbutane. If the halogen is bonded to a simple alkyl group an alternative

Source Image: pubs.acs.org
Download Image
3-Bromo-5-chloro-2-methylaniline | Sigma-Aldrich A pair of theoretical enantiomers is named below. For each stereoisomers, draw the structure. Be sure to specify the stereochemistry via wedge-and-dash bonds. (S)-5,5-dibromo-3-fluoro-2-methyl-3-hexanol and (R)-5,5-dibromo-3-fluoro-2-methyl-3-hexanol; Draw the gauche conformer of the following compound looking down the indicated bond.

Source Image: sigmaaldrich.com
Download Image
2-Bromo-5-chlorohexane | C6H12BrCl | ChemSpider Chemistry Chemistry questions and answers c. Add wedge-and-dash bonds to give (3R)-5-bromo-2-chloro-3-fluoro-3-methylhexane. Indicate the configuration at the 3-position only.
Source Image: chemspider.com
Download Image
5-Bromo-2-chloro-4-fluoro-3-iodopyridine as a Halogen-rich Intermediate for the Synthesis of Pentasubstituted Pyridines | The Journal of Organic Chemistry May 25, 2023c Add wedge and dash bonds to give 3R 5 bromo 2 chloro 3. Last updated: 5/25/2023. c Add wedge and dash bonds to give 3R 5 bromo 2 chloro 3 fluoro 3 methylhexane Indicate the configuration at the 3 position only The configurations at the 2 and 5 positions are unspecified those groups are used to determine priorities for the configuration at the

Source Image: pubs.acs.org
Download Image
1305322-97-7 | 5-bromo-3-chloro-2-fluorophenol – Anax Laboratories c. Add wedgeand-dash bonds to give (3R)-5-bromo-2-chloro-3-fluoro-3-methylhexane. Indicate the configuration at the 3-position only (The configurations at the 2– and 5-positions are unspecified; those groups are used to determine priorities for the configuration at the 3-position.) Not the question you’re looking for?
Source Image: anaxlab.com
Download Image
Search
1305322-97-7 | 5-bromo-3-chloro-2-fluorophenol – Anax Laboratories About Transcript Common and IUPAC nomenclature of alkyl halides. Classifying primary, secondary, and tertiary alkyl halides. Created by Jay. Questions Tips & Thanks Want to join the conversation? Sort by: Top Voted Zoe 11 years ago At about 6:30 , when applying R and S to 2-iodo-3-methylpentane, why isn’t the number 3 carbon chiral as well?
3-Bromo-5-chloro-2-methylaniline | Sigma-Aldrich 5-Bromo-2-chloro-4-fluoro-3-iodopyridine as a Halogen-rich Intermediate for the Synthesis of Pentasubstituted Pyridines | The Journal of Organic Chemistry Chemistry Chemistry questions and answers c. Add wedge-and-dash bonds to give (3R)-5-bromo-2-chloro-3-fluoro-3-methylhexane. Indicate the configuration at the 3-position only.